Over the past 10 months, we have developed novel "mutator" models of cancer in the lung and colon, with the immediate goal of engineering an increased mutational burden within murine cancer. The hypothesis is that an elevated mutational burden will result in an anti-tumor immune response, as seen in human cancer. Ultimately, we envision studying this immune response and using these models to test new immune modulatory therapies for the treatment of cancer. Preliminary studies with these models in the lung, building off of the Kras-LSL-G12D; P53-flox/flox (KP) model developed in this laboratory, are extremely promising. In particular, CRISPR-induced knockout of Msh2 (a DNA mismatch repair gene) in the KP model resulted in a greatly elevated level of mutations across the genome, recapitulating the mutational burden of human cancers harboring mismatch repair deficiency. In addition, immune infiltration is increased in this and other mutational models tested, suggesting that the increased mutational burden is eliciting an immune response, as hypothesized. We are in the process of developing these models in the colon, using the Apc-flox allele as the driving genetic event, as well as breeding all models onto the Rag2-/- background, which lacks an adaptive immune system, to definitively assess the role of the immune system in shaping the mutational landscape of tumors.
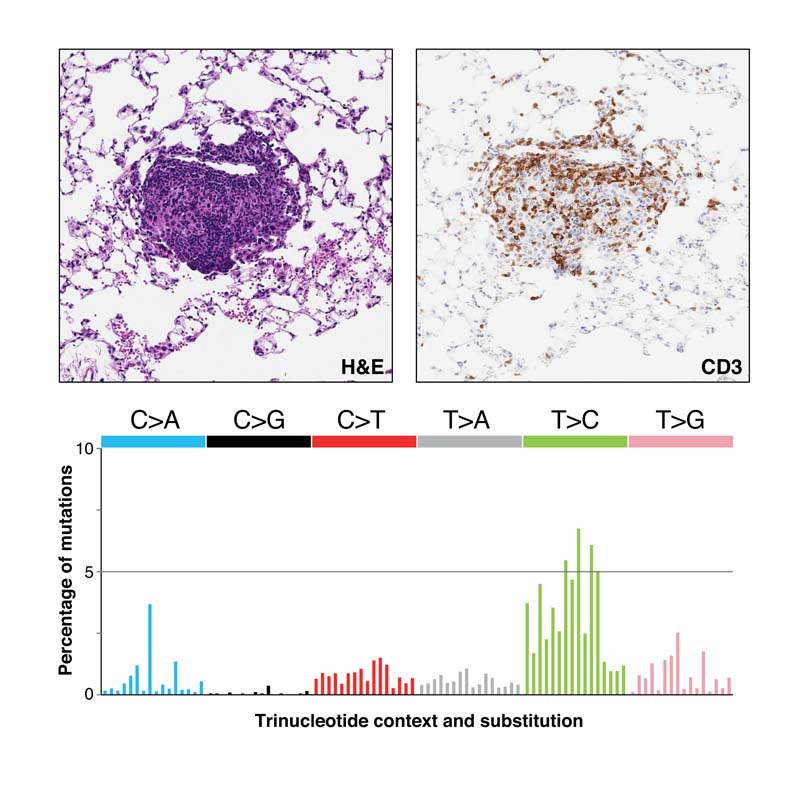
(top) H&E and CD3 staining of a CRISPR-targeted Msh2 knockout lung tumor showing T cell infiltration. (bottom) Mutational spectrum of Msh2 knockout lung tumors recapitulates that seen in human cancers with DNA mismatch repair deficiency.

